Heat Pipes
Description
A heat pipe is a heat exchanger that uses the heat of evaporation of a medium to achieve a high heat flow density. In this way, large amounts of heat can be transported over a small cross-sectional area.The ability of a heat pipe to transport energy depends largely on the specific enthalpy of vaporization of the working medium and not on the thermal conductivity of the vessel wall or working medium. For reasons of efficiency, a heat pipe is usually operated just above the hot end and just below the boiling point of the working medium at the cold end.
Heat pipes are usually elongated metal vessels that contain a hermetically sealed volume. It is filled with a working medium, which fills the volume to a small extent in a liquid state and to a large extent in a gaseous state.
Steam flows to the cooling zone, a film of condensation flows / creeps back. The driving force is the force of adhesion.
Since the vapor and liquid of the working medium are in the same room, the system is located in the wet steam area. As a result, there is exactly a certain temperature in the heat pipe at a certain pressure. Since the pressure differences in heat pipes are very small, usually a few Pascal, the temperature difference between the evaporator and the condenser is also small and is a maximum of a few Kelvin. A heat pipe therefore has a very low thermal resistance. The area between the evaporator and condenser is practically isothermal.
Principle design:

1 = Vapor flow, 2 = Shell, 3 = Heat in, 4 = Heat out, 5 = Liquid flow, 6 = Porous or wick structure made of metal
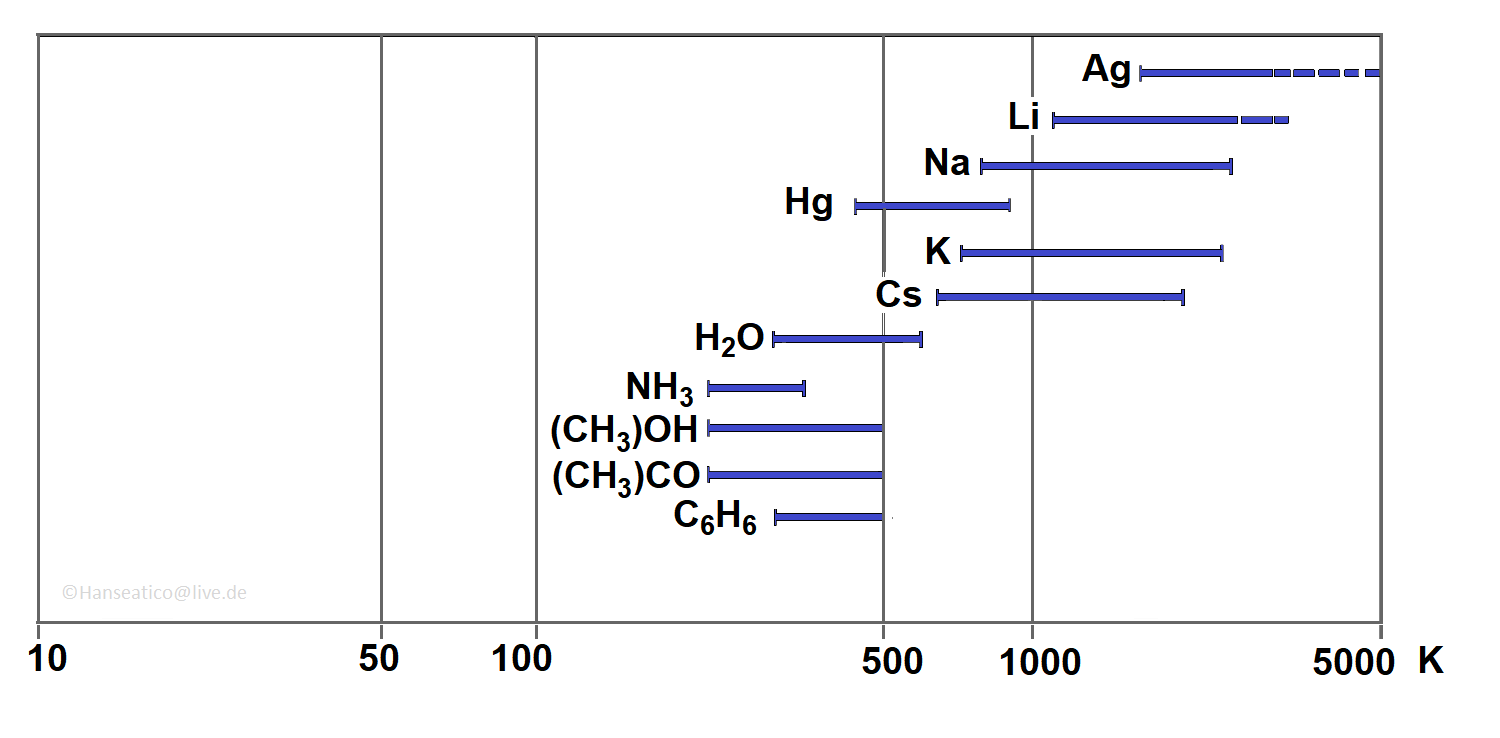
Application temperatures:
Heat Exchanger:

(TU-Dresden, ECEMP - European Centre for Emerging Materials and Processes Dresden)
[Click elsewhere on the background of the main window to close this popup]